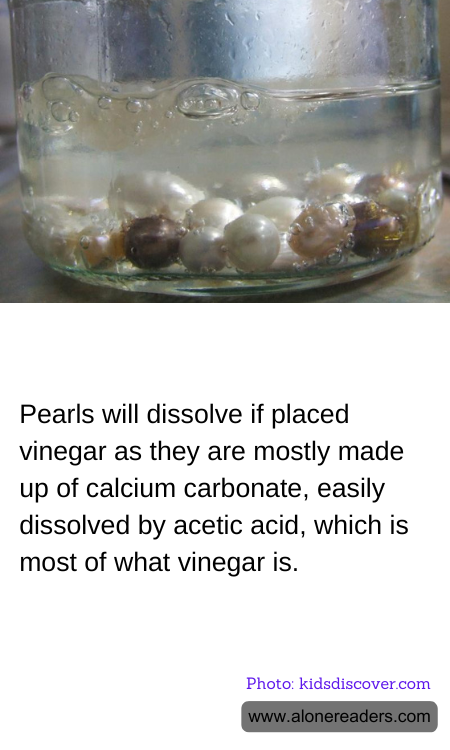
Pearls have long been admired for their natural beauty and elegance, often symbolizing purity and sophistication. These lustrous gems are formed within the soft tissue of a living shelled mollusk, such as an oyster or a mussel. The primary component of pearls is calcium carbonate, which is also found in limestone, marble, and the shells of many marine organisms. This mineral composition gives pearls their distinctive sheen and durability. However, it also makes them susceptible to certain chemical reactions, particularly with acids.
One of the most intriguing interactions involving pearls is their reaction with vinegar. Vinegar, a common household item, is primarily composed of acetic acid. When pearls are placed in vinegar, a fascinating chemical reaction occurs. The acetic acid in the vinegar reacts with the calcium carbonate in the pearls, leading to the dissolution of the pearls over time. This reaction can be likened to the fizzing and bubbling that occurs when baking soda is mixed with vinegar, albeit at a slower pace.
The science behind this reaction is relatively straightforward. Calcium carbonate, the main constituent of pearls, is a base. When it comes into contact with an acid like vinegar, a neutralization reaction occurs. This results in the formation of calcium acetate, water, and carbon dioxide gas. The release of carbon dioxide gas is what causes the fizzing effect, and over time, the pearl will gradually dissolve as more and more of its calcium carbonate is converted into calcium acetate.
This phenomenon is not just a curious scientific fact but also serves as a reminder of the delicate nature of pearls. Despite their outward appearance of strength and resilience, pearls require careful handling and maintenance to preserve their beauty. Exposure to acidic substances, even those as mild as vinegar, can cause irreversible damage. This is why it is recommended to avoid contact with acidic foods, perfumes, and other harsh chemicals when wearing pearl jewelry.
For those who own pearls, understanding their chemical composition and the potential risks posed by acids can help in maintaining their luster and longevity. Simple care tips, such as wiping pearls with a soft cloth after wearing them and storing them separately from other jewelry, can go a long way in preserving their natural beauty. Additionally, it is advisable to have pearls restrung periodically, as the silk thread that holds them together can weaken over time.
In conclusion, while the interaction between pearls and vinegar is a captivating example of chemistry in action, it also underscores the importance of proper care for these precious gems. By being mindful of the substances that come into contact with pearls, one can ensure that their elegance and allure endure for generations to come.