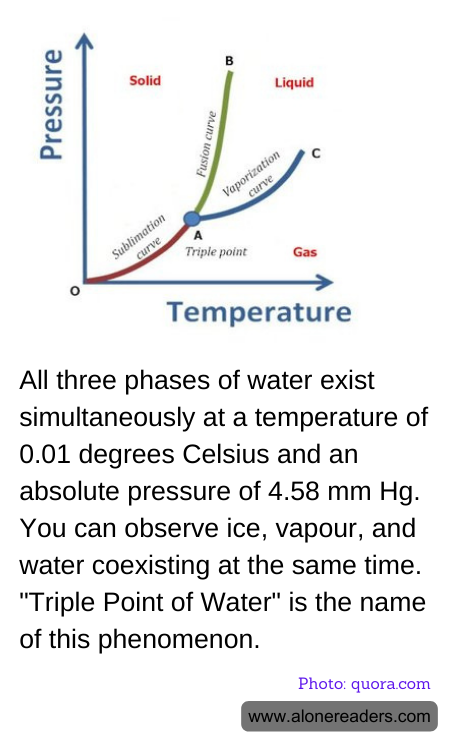
The Triple Point of Water is a fascinating scientific phenomenon where water's three phases—solid, liquid, and gas—exist in equilibrium. This unique condition occurs at a specific temperature and pressure, precisely at 0.01 degrees Celsius and an absolute pressure of 4.58 mm Hg. At these exact conditions, ice, water vapor, and liquid water coexist in a stable state, demonstrating a delicate balance between the different phases of matter.
Understanding the Triple Point requires a grasp of basic thermodynamics and phase transitions. Normally, water transitions from ice to liquid, or from liquid to vapor, depending on the ambient temperature and pressure. However, at the Triple point, the energy states of solid, liquid, and gaseous water are such that they can seamlessly transition from one to another without changing the overall temperature or pressure.
This phenomenon is not only a curious scientific oddity but also has practical implications in fields like meteorology and materials science. In meteorology, understanding the conditions under which water can exist in all three states is crucial for predicting weather patterns and phenomena like frost or dew. In materials science, the principles of the Triple Point help in studying the properties of materials at various temperatures and pressures.
The concept of the Triple Point also serves as an important standard in the calibration of thermometers and in defining the units of temperature. Until recently, the Triple Point of water was used to define the Kelvin, the base unit of temperature in the International System of Units (SI). Scientists use high-precision equipment to replicate the exact conditions of the Triple Point to ensure temperature measurements are accurate and consistent across different instruments and laboratories.
In summary, the Triple Point of water is more than just a scientific curiosity. It illustrates the intricate balance of nature's forces and serves crucial practical purposes in science and technology. Witnessing water in all three states simultaneously underlines the dynamic and extraordinary properties of this commonplace yet remarkable molecule.