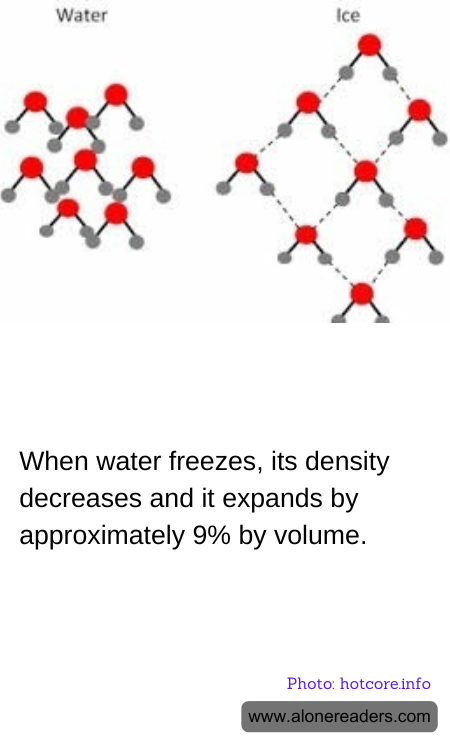
Water exhibits a unique behavior compared to most other substances due to its molecular structure. When water freezes into ice, its density decreases, which is quite contrary to typical substances that become denser as they solidify. The reason behind this unusual property lies in the arrangement of water molecules.
In liquid form, water molecules are closely packed, but they move freely and randomly. As the temperature decreases, the kinetic energy of the molecules reduces, slowing down their movement and increasing the interactions between the hydrogen bonds. These bonds are critical in defining water's properties. As water approaches its freezing point, these hydrogen bonds arrange water molecules into a structured, hexagonal lattice that maximizes the space between them.
This hexagonal arrangement results in ice having a more open structure than liquid water. Consequently, ice occupies more volume than the same amount of water, leading to a density decrease. This increase in volume is approximately 9%, which explains why ice floats on water. This is a crucial factor in aquatic ecosystems, especially in polar regions. If ice were denser than water, lakes, seas, and oceans would freeze from the bottom up, drastically altering aquatic habitats and making life in these environments nearly impossible during periods of freezing temperatures.
The floating ice acts as an insulating barrier on water bodies, preventing the water underneath from freezing and allowing life to exist even in extreme cold. Thus, the anomalous expansion of water not only influences the behavior of water in various physical contexts but also plays a pivotal role in sustaining life in cold climates. This unusual property of water is one of the many reasons it is essential to life on Earth.