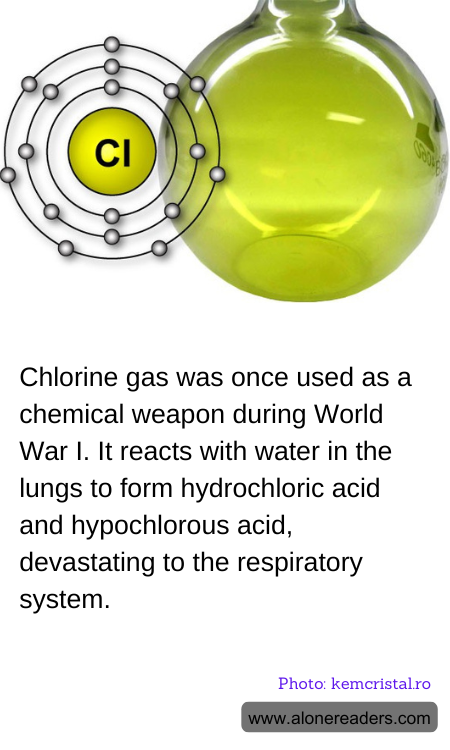
Chlorine gas, a toxic chemical, was first used as a weapon during World War I, marking a grim milestone in the history of warfare. Its deployment on the battlefield was a result of its potent properties as a pulmonary irritant, designed to incapacitate and demoralize enemy troops. When chlorine gas comes into contact with moist tissues such as those found in the human lungs, it undergoes a chemical reaction with the water present to produce hydrochloric acid and hypochlorous acid.
This reaction severely damages the respiratory system by corroding the lung tissue, leading to acute respiratory distress and sometimes death. The gas, which is heavier than air, would cling low to the ground and could easily fill the trenches, making it particularly effective and horrifying as a weapon. The release of chlorine causes choking, severe eye and skin irritation, and it can leave victims with permanent respiratory damage if they survive the initial exposure.
The introduction of chlorine gas in warfare led to the development of gas masks and other protective measures, which slowly evolved into more sophisticated chemical and biological war defense systems. The ethical implications of using such a devastating weapon sparked international outrage, ultimately contributing to the development of the Geneva Protocol in 1925, which prohibits the use of chemical and biological weapons in war. However, the legacy of chemical warfare and the usage of chlorine gas during World War I underscore the tragedies that result when scientific advancements are harnessed for destructive purposes.
In modern times, the use of chlorine is strictly regulated under various international agreements to prevent its misuse, reflecting a global commitment to preventing the horrors of chemical warfare from recurring. Despite this, chlorine remains a potent reminder of the destructiveness of war and the enduring need for vigilance in the control of chemical substances.