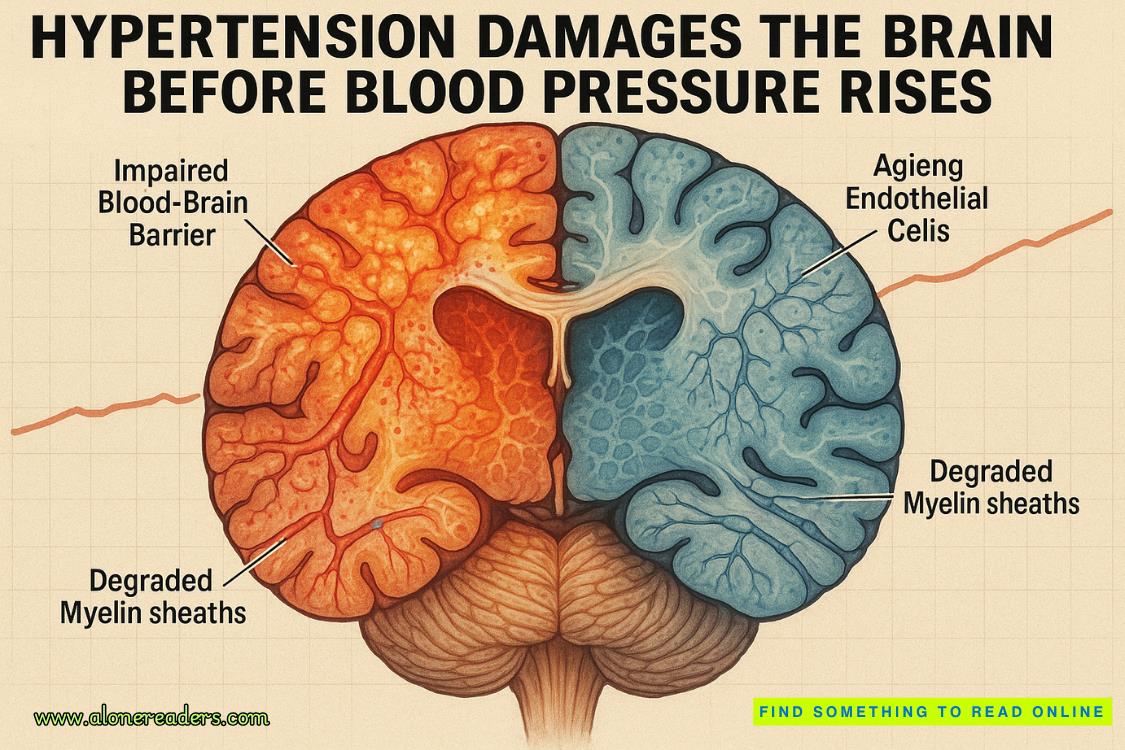
Emerging evidence is forcing a rethink: the commonly held assumption that brain damage from high blood pressure (hypertension) occurs only after the blood-pressure reading crosses a specific threshold is no longer tenable. A recent preclinical study from the Weill Cornell Medicine team shows that brain injury at the level of gene-expression and cellular dysfunction can precede any measurable rise in systemic blood pressure. Broadly speaking, the damage doesn’t wait for the red zone on the sphygmomanometer—it starts much earlier, under the radar. This article delves into the molecular and cellular mechanisms at play, explores how these early changes translate into cognitive risk, and considers what it means for prevention and clinical practice.
Zeroing in on early changes. Conventional wisdom: persistent elevated blood pressure damages the vasculature and thereby the brain. In contrast, the new findings show that within as few as three days of inducing hypertension in animal models (via angiotensin-II administration), key brain cell types already display aging and dysfunction—even before any sustained rise in blood pressure is observed. This was demonstrated through single-cell gene-expression profiling, showing that: endothelial cells, interneurons and oligodendrocytes all had significant transcriptomic alterations.
Why this matters. Because if damage begins before the blood pressure crosses diagnostic thresholds, waiting for “hypertension” to be established may mean missing a window of opportunity. The brain appears to be primed for injury by vascular or neurovascular stressors tied to the initial perturbation—even absent frank hypertension.
Endothelial cells – the gatekeepers go awry. Endothelial cells lining brain capillaries begin to exhibit signs of senescence, reduced mitochondrial/energy-metabolism gene expression, and dysfunctional responses within days. The blood-brain barrier (BBB) shows early evidence of compromise, meaning that harmful plasma components, inflammatory mediators or metabolic waste may access brain parenchyma when they shouldn’t. This reduction in BBB integrity is a key early event.
Interneurons – the signalling disruptors. Interneurons regulate and balance excitatory and inhibitory signals in cortical networks. The research shows that these cells manifest gene-expression changes associated with altered inhibitory neurotransmission, early synaptic dysfunction and network imbalance. The result: even before large-scale neuronal death, circuit dysfunction sets in—analogous to what is seen in early Alzheimer’s or vascular cognitive impairment.
Oligodendrocytes & white-matter maintenance – the communication breakdown. Oligodendrocytes produce myelin sheaths, which are critical for rapid signal conduction. Early hypertension induction reduced expression of genes governing oligodendrocyte maintenance, myelin repair and turnover. The implication: white-matter tracts become vulnerable—and communication between brain regions begins to degrade.
Timing and progression. In the cited study, at day 3 after angiotensin-II infusion (but before BP rose) these changes were already present; by day 42, when BP was elevated and cognitive decline measurable, further gene-expression changes and structural deficits had emerged. So the process is sequential: early cellular vulnerability → worsening vascular/neurovascular injury → measurable hypertension + clinical cognitive decline.
Linking early injury with long-term risk. Patients with hypertension have a 1.2- to 1.5-fold higher risk of developing cognitive disorders such as vascular cognitive impairment or even Alzheimer’s disease, compared to normotensive individuals. Importantly, many antihypertensive treatments effectively lower BP yet fail to fully prevent cognitive decline—suggesting that some of the brain injury may occur independently of the achieved BP levels.
Mechanisms of transition. The early endothelial damage leads to impaired cerebral autoregulation, reduced cerebrovascular reserve, and accumulation of microvascular lesions and white-matter hyperintensities over time. Interneuron dysfunction and compromised myelin exacerbate network inefficiency and synaptic fatigue. Together these lead to slower processing speed, executive-function decline, memory impairment and ultimately a higher likelihood of dementia.
Why blood pressure control alone may not suffice. While BP lowering remains essential, the fact that brain injury precedes hypertension means that by the time the BP is high and treated, some damage may already be “locked in.” In such cases, reducing BP may halt further injury, but might not reverse early damage. This helps explain why certain large clinical trials of aggressive BP lowering show only modest cognitive benefit.
Screening and risk stratification shifted earlier. Clinicians may need to identify individuals at risk—those with pre-hypertensive BP readings, signs of vascular stress (e.g., elevated pulse-wave velocity, arterial stiffness), or early endothelial dysfunction—and consider brain-health monitoring or neurovascular protective strategies before full hypertension sets in.
Beyond BP: neurovascular protection. The findings point to a new therapeutic dimension: protecting the neurovascular unit (endothelium + oligodendrocytes + neurons) rather than focusing solely on systemic BP. For example, angiotensin-receptor inhibitors (ARBs) such as Losartan reversed early endothelial and interneuron changes in the animal study. This suggests that choice of antihypertensive agent may matter for brain health beyond BP lowering.
Lifestyle and systemic-vascular health as early modulators. Because the earliest changes involve endothelial aging, metabolic dysfunction and vascular stress, key preventive measures include: maintaining healthy vascular function (via exercise, diet, smoking cessation), reducing oxidative-inflammatory burden, ensuring good sleep (since sleep disturbance influences vascular/neurovascular health), controlling diabetes, lipids and obesity—and perhaps assessing and treating subclinical vascular dysfunction even in borderline BP.
Imaging and biomarker surveillance. In higher-risk individuals, advanced neuroimaging (white-matter integrity, microvascular changes) or emerging biomarkers of endothelial senescence/vascular aging might become relevant. Monitoring brain health in “pre-hypertensive” patients may identify those who would benefit from early intervention.
Clinical roadmap:
Research directions:
The old paradigm—that brain injury from hypertension begins only when blood pressure crosses the clinical threshold—is being overturned. The new paradigm: vascular and neurovascular injury begins early, at the level of endothelial aging, interneuron and oligodendrocyte dysfunction, before any measurable elevation in blood pressure. Recognising this means shifting our focus upstream: from simply controlling systemic BP to proactively monitoring and protecting the brain’s vascular-neural ecosystem. For the technically minded clinician or researcher, the imperative is to treat the brain as a target organ from the very first signs of vascular stress—not just after the damage is done. Early intervention, smart choice of therapies, and a holistic vascular-neurological mindset may offer the best chance to prevent irreversible cognitive decline.